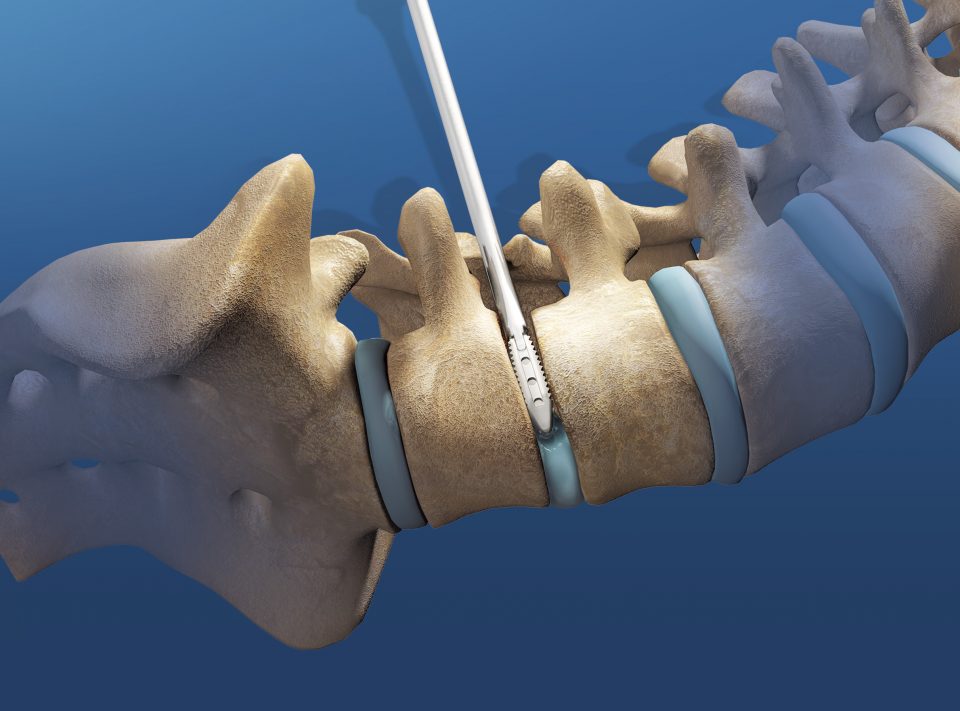
Indications
The FORZA Spacer System is indicated for spinal fusion procedures in skeletally mature patients with degenerative disc disease (DDD) at one or two contiguous levels in the lumbar spine (L2-S1). DDD is defined as back pain of discogenic origin with degeneration of the disc confirmed by patient history and radiographic studies. DDD patients may also have up to Grade 1 spondylolisthesis at the involved levels. These patients may have had a previous non-fusion surgery at the involved level(s).
The FORZA Spacer System is intended for use with autograft and supplemental fixation. As an example, the supplemental fixation that may be used is the Orthofix Firebird™ Pedicle Screw System.
Patients must have undergone a regimen of at least six (6) months of non-operative treatment prior to being treated with the FORZA Spacer System.
Contraindications
The FORZA Spacer System, as with other orthopedic implants, is contraindicated for use in patients:
- With active infections in which the use of an implant could preclude adequate and appropriate treatment of the infection.
- Who have had prior fusion at the level to be treated.