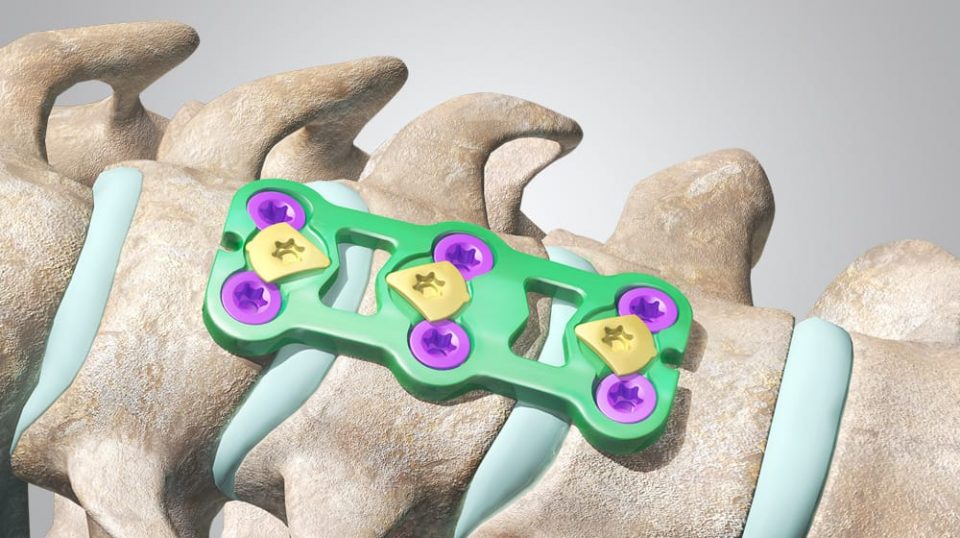
Indications
The CETRA Anterior Cervical Plate System is intended for anterior fixation to the cervical spine from C2 to C7. The specific clinical indications include:
- Degenerative disc disease (as defined as back pain of discogenic origin with degenerative disc confim1ed by patient history and radiographic studies)
- Spondylolisthesis
- Trauma
- Spinal stenosis
- Deformities (i.e., scoliosis, kyphosis, and/or lordosis)
- Tumor
- Pseudoarthrosis
- Revision of previous surgery
Contraindications
The CETRA Anterior Cervical Plate System is contraindicated in patients with a systemic infection, with a local inflammation at the bone site, or with rapidly progressive joint disease or bone absorption syndromes such as Paget’s disease, osteopenia, osteoporosis, or osteomyelitis. Do not use this system in patients with known or suspected metal allergies. Use of the system is also contraindicated in patients with any other medical, surgical or psychological condition that would preclude potential benefits of internal fixation surgery such as the presence of tumors, congenital abnormalities, elevation of sedimentation rate unexplained by other disease, elevation of white blood cells or a marked shift in white blood cell differential count.