- Fenestrated screw design allows bone growth through the implant
- Low profile screw head designed to prevent soft tissue irritation
- Indicated for autograft and allograft with instruments designed to deliver additional biologic material into the SI joint
- Incorporates radial slots along the screw’s body intended to optimize surrounding bone access to the bone substitute, allowing greater bone growth through the SambaScrew
- Tapered screw tip to aid in guidance through pilot hole
- Low profile screw head prevents soft tissue irritation
- Available in 6 lengths to accommodate patient anatomy, 25mm-50mm in 5mm increments
SambaScrew SI Fixation System
The SambaScrew® System is a complete solution to fixating the sacroiliac joint. It consists of a 9mm diameter, cannulated screw with multiple fenestrations on its shaft with streamlined instrumentation.

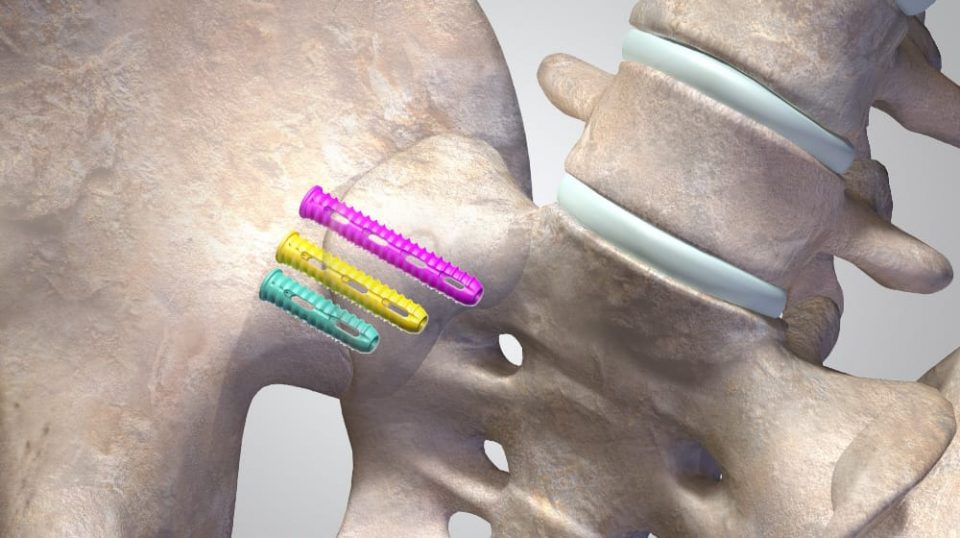
Indications
The SambaScrew SI Fixation System is intended for fixation of sacroiliac joint disruptions. This fixation device is to only be used in skeletally mature patients.
Contraindications
The SambaScrew SI Fixation System is contraindicated for use in patients with:
Open wounds, infection, presence of tumor, pregnancy, osteoporosis, certain metabolic disorders affecting osteogenesis, certain inflammatory/neuromuscular conditions, and certain neuromuscular deficits which would place an unusually heavy load on the device during the healing period.
The implant is made from Ti-6AI-4V ELI (medical-grade titanium alloy). The fixation implant is contraindicated in any individual with a known or suspected allergy, sensitivity or intolerance to metal.