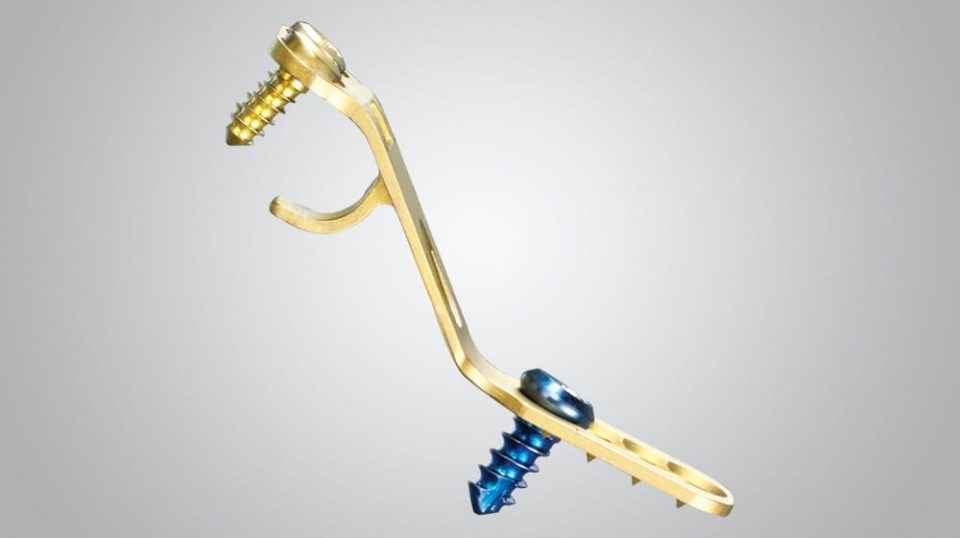
Indications
The NewBridge Laminoplasty Fixation System is indicated for use in the lower cervical and upper thoracic spine (C3-T3) after a laminoplasty has been performed. The NewBridge Laminoplasty Fixation System holds or buttresses the allograft in place in order to prevent expulsion of the allograft or impingement of the spinal cord.
Contraindications
The NewBridge Laminoplasty Fixation System is not to be used:
- For screw attachment or fixation to the posterior elements of the lumbar spine
- For single or two level spondylosis without developmental spinal canal stenosis
- Under any direct load bearing conditions
The NewBridge Laminoplasty Fixation System is not to be used when there is:
- Focal anterior compression
- Isolated radiculopathy
- Loss of anterior column support resulting from tumor, trauma, or infection
The NewBridge Laminoplasty Fixation System must always be used with an allograft.