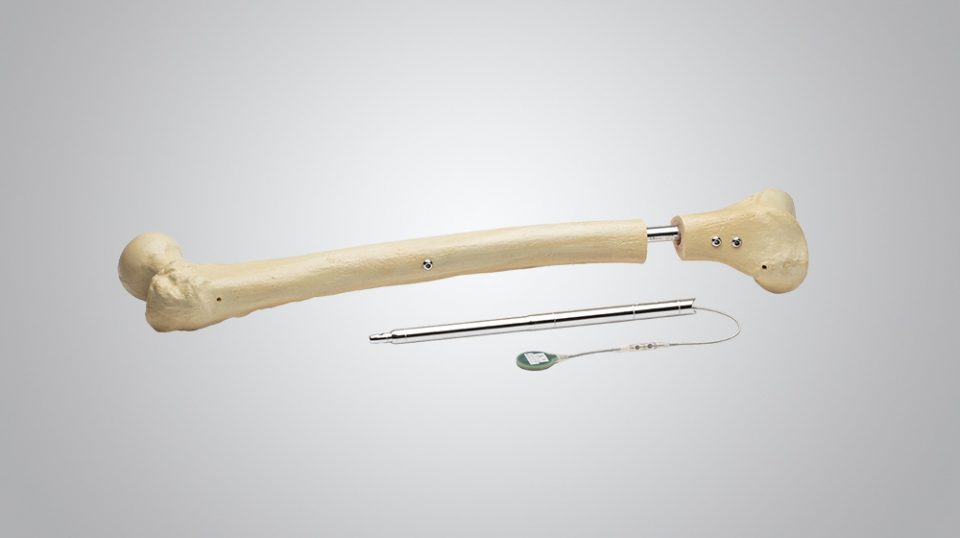
Benefits to Surgeon
Precision
- Instruments designed not only for lengthening but for optimal limb alignment
- Protection of soft-tissues with minimally-invasive instrumentation
- Safe alignment assessment and blocking screw placement with dummy (trial) nail
Power
- Reliable power direct to the nail
- High distraction force independent of nail size
- No soft-tissue limitations
- Power transferred to the nail with small portable Control Set
- No magnets within the control set
Fit
- Bone is reamed to exactly fit the implant (step reamer)
- No over-reaming necessary, therefore it is a bone-conserving procedure
Manufacturer information is available on the product labels and relevant IFUs.