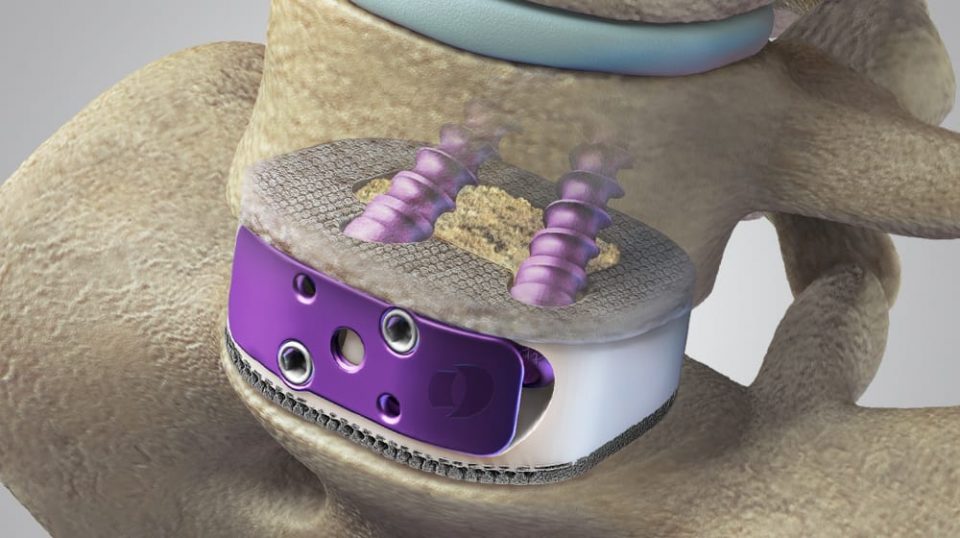
Indications
The PILLAR SA PTC Spacer System is indicated for spinal fusion procedures in skeletally mature patients with Degenerative Disc Disease (DDD) at one or two contiguous levels in the lumbar spine (L2-S1). DDD is defined as back pain of discogenic origin with degeneration of the disc confirmed by patient history and radiographic studies. DDD patients may also have up to Grade 1 spondylolisthesis at the involved levels. These patients may have had a previous non-fusion surgery at the involved level(s).
The PILLAR SA PTC Spacer System is intended for use with autograft and/or allograft comprised of cancellous and/or corticocancellous bone graft.
The PILLAR SA PTC Spacer System is intended for use with four of the titanium alloy screws provided with the system. If the physician chooses to use fewer than four of the provided screws then supplemental fixation must be used to augment stability. As an example, a supplemental fixation system that may be used is the Orthofix Firebird® Spinal Fixation System.
Patients must have undergone a regimen of at least six months of non-operative treatment prior to being treated with the PILLAR SA PTC Spacer System.
Contre-indications
The PILLAR SA PTC Spacer System, as with other orthopedic implants, is contraindicated for use in patients with:
- Active infections in which the use of an implant could preclude adequate and appropriate treatment of the infection.
- Rapidly progressive joint disease or bone absorption syndromes such as Paget’s disease, osteopenia, osteoporosis, or osteomyelitis which may prevent adequate fixation.
- Conditions that may place excessive stresses on bone and implants, such as severe obesity, pregnancy or degenerative diseases. The decision to use this system in such conditions must be made by the physician taking into account the risks versus the benefits to the patient.
- Prior fusion at the level to be treated.
- Any circumstances not listed under the heading indications.